What Is the Charge on the Iron Ion in Fe2s3
The charges of all the atoms must add up to 0. Each iron ion has a 3 charge as 623.

What Is The Charge On The Iron Ion In Fe2s3 Brainly Com
When you write a formula unit for a salt compound it too must be uncharged.

. Webew7 and 6 more users found this answer helpful. Posted by Kimo Khaled. The charge of each iron ion in the ionic compound FeS is Fe2.
For FeS the iron is FeII and for the Fe2S3 the iron is FeIII. Again no numerical prefixes appear in the name. What is the most stable state of an iron ion.
Anions 1-acetate C 2 H 3 O 2-cyanide CN-amide NH 2-cyanate OCN-hydrogen carbonate fluoride F. 0 followers 0 following Joined October 2021. Is Fe2S3 iron II or III.
As the molecule has a neutral charge we know the sum of the. To neutralise a -6 charge a 6 charge is required. Each sulfide ion has a 2- charge so if there are three sulfide ions the two iron cations must have a total charge of 6 or 3 on each cation.
Step 1 of 4. Based on the formula there are two iron cations and three sulfide ions. As there is only one iron ion its charge must be 2.
If the metal forms only a single type of cation no Roman numeral is used to indicate the charge. These ions are charged but the solid crystal is not because the ions are arranged in a specific proportion. Roman numeral notation indicates charge of ion when element commonly forms more than one ion.
The iron cation in Fe2S3 has a 3 charge. Hope this helps pls mark brainliest D. The iron cation in Fe2S3 has a 3 charge.
It is an ironII ion as opposed to ironIII ion which has a charge of 3. See full answer below. The bond between H and O is polar covalent.
Therefore this compound is known as known as iron III sulfate or ferric sulfate with the number referring to. What is the ionic charge of an ion with 13 protons and 10 electrons. For example ironII has a 2 charge.
For monoatomic cation the name is same as the element name. May 14 2021 3 Each ion of iron Fe in Fe 2 S3 is carrying a charge of 3. A black powder it decomposes at room temperature to give iron 2 chloride hydrogen sulfide and sulfur.
For FeS the iron is Fe II and for the Fe2S3 the iron is Fe III. What is the charge on the iron ion in Fe2S3. The name of the compound Fe2S3 is ironII sulfide.
Home Community What is the most stable state of an iron ion. An anion has more electrons than protons consequently giving it a net negative charge. To balance the oxidation state of Fe3S4 requires a mixture of FeS and Fe2S3.
The correct formula for iron II bromide is FeBr2. Each sulfide ion has a 2- charge so if there are three sulfide ions the two iron cations must have a. Up to 256 cash back what is the charge on the iron ion in fe2s3.
The common ions of iron are FE and FE2 False. Based on the formula there are two iron cations and three sulfide ions. Hence FeS is ironII sulfide while Fe 2 S 3 is ironIII sulfide.
In an ionic molecule the formula is determined by balancing the charges on the ions. CID 23925 FE III Ion CID 29109 Sulfide Date s. For this case the roman numeral III tells us the iron has a charge of 3 or Fe3 The the element sulfur has a charge of -2 or S-2.
Ammonia NH3 is a polar molecule. 3 sulfate ions have a charge of -6 as -2 is multiplied by 3. The molecule iron III sulfide is an ionic molecule between the metal Iron and the non-metal sulfur.
The name of a binary ionic compound is given as the cation name first followed by anion name. IronIII a 3 charge. Downvote Inorganic chemistry Iron Ions Science Chemistry Stability.
If the metal forms more than one type of cation Roman. Oxygen in nearly all cases has a charge of 2 - except for a few compounds such as potassium superoxide K O2 so the total ionic charge the O atoms exhibit is 3 - 2 - 6. IronIII oxide is a neutral compound which implies the net charge of the compound equals 0.
Each ion of iron Fe in Fe2 2 S3 3 is carrying a charge of 3. In naming ionic compounds whose cations can have more than one possible charge we must also include the charge in parentheses and in roman numerals as part of the name. The Roman numeral II indicates the iron ion has a 2 charge.
Iron 3 sulfide is a member of the class of iron 3 sulfides that has formula Fe2S3.
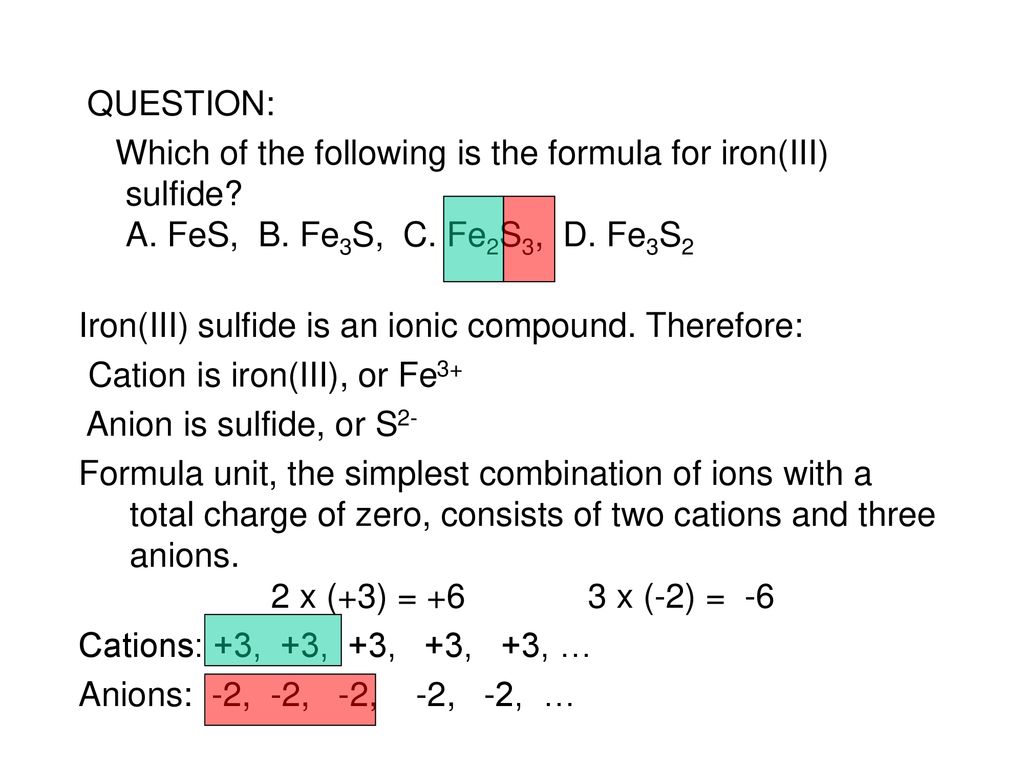
Iron Iii Sulfide Is An Ionic Compound Therefore Ppt Download

Comments
Post a Comment